 RFID (Radio Frequency Identification) technology has been around for many years. It first caught on in retail and logistics as a natural fit. Items with RFID labels, also referred to as Smart Labels or Intelligent Labels, can be tracked and identified efficiently through every stage of the supply chain, from purchasing to shipping to delivery.
RFID (Radio Frequency Identification) technology has been around for many years. It first caught on in retail and logistics as a natural fit. Items with RFID labels, also referred to as Smart Labels or Intelligent Labels, can be tracked and identified efficiently through every stage of the supply chain, from purchasing to shipping to delivery.
Why is RFID a good solution for medical devices?
Consider the FDA requirements that mandate unique device identification (UDI) for medical devices. With a UDI system already in place, barcodes can become smart codes by having RFID sensors embedded in the barcode labels. RFID sensors can be engineered to withstand the high temperatures of sterilization, and by using thermal data logging technologies, hospitals can track assets through use, sterilization, and reuse.
Comprising of a small chip, which is capable of carrying up to 2000 bytes and an antenna, the RFID devices do a job similar to that of a bar code or a magnetic strip on the back of a credit card or ATM card – provide a unique identifier for an object. Like a bar code or magnetic strip, an RFID device must also be scanned to gather the identifying information.
 Today, pharmaceutical companies are also recognizing the benefits of RFID labels, and for very good reasons. In pharma, RFID labels help to manage inventory, increase operational efficiencies, remain in compliance with governmental regulation, and ensure the wellbeing of patients and consumers. RFID labels also enable itemizing and sorting of stock to ensure quality and prevent waste.
Today, pharmaceutical companies are also recognizing the benefits of RFID labels, and for very good reasons. In pharma, RFID labels help to manage inventory, increase operational efficiencies, remain in compliance with governmental regulation, and ensure the wellbeing of patients and consumers. RFID labels also enable itemizing and sorting of stock to ensure quality and prevent waste.
RFID within the healthcare segment helps make medicine and assets smarter by providing improved visibility of inventory and assets. It's true that lost or stolen materials cost the healthcare industry millions every year. With RFID labels, you can see medicines and devices travel through the supply chain as intended, providing an accurate chain of custody. Improved visibility reduces the risk of counterfeit drugs entering legitimate distribution channels and unintended product diversion. Accurate, timely and complete data helps avoid product expiration and stock outages and effective recall management.
Nothing is more important than patient safety, but counterfeit drugs are real and one of the largest fraudulent markets in the world. Radio-frequency identification can help provide a quick way to retrieve information, track pharmaceuticals or items in the supply chain, and help avoid the costs associated with counterfeit or adulterated medications. The tag is placed on an individual object, which allows for unique identification.
Efforts of the FDA, in collaboration with pharmaceutical suppliers, to maintain a secure drug supply in the wake of rising counterfeit drugs prevalence, has encouraged the pharmaceutical industry to use RFID in combination with the electronic product code (EPC) for real-time tracking, tracing, and authentication of drugs.
 It is critical to provide the right drug and of course, one that's free of tampering. RFID not only ensures integrity, it delivers strong counterfeiting deterrence as the label itself could be used to authenticate the pharmaceutical product and tamper evidence.
It is critical to provide the right drug and of course, one that's free of tampering. RFID not only ensures integrity, it delivers strong counterfeiting deterrence as the label itself could be used to authenticate the pharmaceutical product and tamper evidence.
The U.S. Food and Drug Administration (FDA) has played a vital role in escalating the widespread adoption of Radio Frequency Identification (RFID) in the pharmaceutical sector to strengthen anti-counterfeiting and track-and-trace efforts. Besides these benefits, RFID technology is acknowledged for its ability to increase supply chain efficiency, reduce errors by proper storage of information, boost patient safety and monitoring, enhance staff, patient, and asset workflow, easy adoption and flexible usage, and cut down labor requirements.



 Over the years since Walmart has continued to work with
Over the years since Walmart has continued to work with 
 RFID (Radio Frequency Identification) technology has been around for many years. It first caught on in retail and logistics as a natural fit. Items with RFID labels, also referred to as Smart Labels or Intelligent Labels, can be tracked and identified efficiently through every stage of the supply chain, from purchasing to shipping to delivery.
RFID (Radio Frequency Identification) technology has been around for many years. It first caught on in retail and logistics as a natural fit. Items with RFID labels, also referred to as Smart Labels or Intelligent Labels, can be tracked and identified efficiently through every stage of the supply chain, from purchasing to shipping to delivery. Today, pharmaceutical companies are also recognizing the benefits of RFID labels, and for very good reasons. In pharma, RFID labels help to manage inventory, increase operational efficiencies, remain in compliance with governmental regulation, and ensure the wellbeing of patients and consumers. RFID labels also enable itemizing and sorting of stock to ensure quality and prevent waste.
Today, pharmaceutical companies are also recognizing the benefits of RFID labels, and for very good reasons. In pharma, RFID labels help to manage inventory, increase operational efficiencies, remain in compliance with governmental regulation, and ensure the wellbeing of patients and consumers. RFID labels also enable itemizing and sorting of stock to ensure quality and prevent waste.  It is critical to provide the right drug and of course, one that's free of tampering. RFID not only ensures integrity, it delivers strong counterfeiting deterrence as the label itself could be used to authenticate the pharmaceutical product and tamper evidence.
It is critical to provide the right drug and of course, one that's free of tampering. RFID not only ensures integrity, it delivers strong counterfeiting deterrence as the label itself could be used to authenticate the pharmaceutical product and tamper evidence.  We have all seen RFID labels and tags on products in stores. Usually they are on more expensive items that are small and are often targets of shoplifting. `
We have all seen RFID labels and tags on products in stores. Usually they are on more expensive items that are small and are often targets of shoplifting. `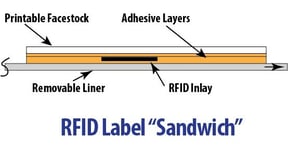 RFID (Radio Frequency Identification) is an automatic identification method that stores and remotely retrieves data via an RFID inlay embedded in a label or tag. The components of an RFID label include:
RFID (Radio Frequency Identification) is an automatic identification method that stores and remotely retrieves data via an RFID inlay embedded in a label or tag. The components of an RFID label include:




